Element Number 85: Unraveling the 7 Little Words Mystery
Element number 85, also known as astatine, is the answer to the 7 Little Words clue. But there’s much more to this element than just a simple game solution. This radioactive element, a member of the halogen family, holds a fascinating story of discovery, properties, and potential applications. Let’s delve into the world of element number 85 and uncover its secrets.
What is Element Number 85 (Astatine)?
Astatine is a highly radioactive, synthetic element, meaning it’s not found naturally in significant amounts. It exists only briefly as a product of the decay of heavier elements like uranium and thorium. Due to its short half-life, even the most stable isotope of astatine lasts only a few hours. This makes studying its properties a real challenge. Understanding its existence is crucial to grasping the larger picture of radioactive elements and their behavior.
The Elusive Nature of Astatine
The discovery of astatine was a collaborative effort, with credit given to Dale R. Corson, Kenneth Ross MacKenzie, and Emilio Segrè at the University of California, Berkeley, in 1940. They bombarded bismuth with alpha particles, successfully creating element 85. Its name, derived from the Greek word “astatos,” meaning unstable, reflects its fleeting existence. This instability makes astatine one of the rarest elements on Earth.
Astatine’s rarity and radioactivity limit its practical applications. However, its potential use in targeted alpha-therapy for cancer treatment is being actively investigated. The ability of astatine to deliver targeted radiation to cancerous cells holds promise for more effective and less harmful cancer therapies. This is an area of ongoing research, with scientists exploring different astatine isotopes and delivery methods.
Exploring the Properties of Element Number 85
Astatine shares similarities with other halogens, like iodine, but its radioactive nature gives it unique characteristics. While its chemical properties can be predicted based on its position in the periodic table, experimental verification remains difficult due to its instability. Despite these challenges, researchers continue to explore its behavior.
Astatine’s Role in Nuclear Medicine
Although astatine’s radioactivity presents challenges, it also opens doors for potential medical applications. The focused emission of alpha particles makes it a potential candidate for targeted alpha-therapy, aiming to destroy cancer cells while minimizing damage to surrounding healthy tissue. This precision is key to improving cancer treatment outcomes.
 Astatine in Targeted Alpha-Therapy
Astatine in Targeted Alpha-Therapy
Understanding the Decay Chain of Astatine
Astatine undergoes radioactive decay, transforming into other elements through alpha and beta decay processes. Understanding this decay chain is essential for managing its use in medical applications and for comprehending its role in the natural environment. The decay products also contribute to the overall radioactivity and need to be considered in any application.
Element Number 85 and 7 Little Words: The Connection
The clue “element number 85” in 7 Little Words is a clever way to introduce players to this fascinating element. While the answer is astatine, the clue sparks curiosity and encourages players to learn more about this elusive element. This playful introduction to a scientific concept is a testament to the game’s educational value.
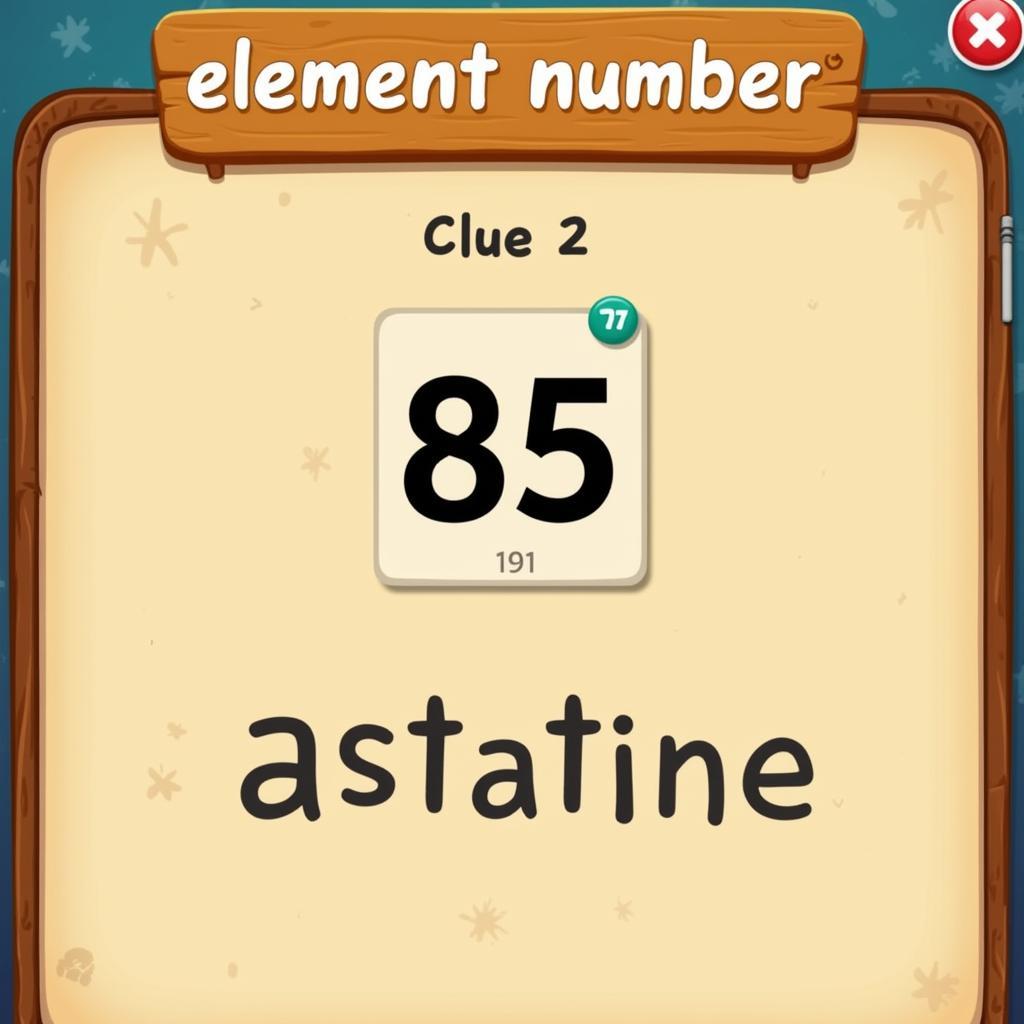 7 Little Words Puzzle Featuring Astatine
7 Little Words Puzzle Featuring Astatine
Conclusion: Element number 85, astatine, remains a subject of ongoing research. Its rarity and radioactivity present challenges, but also exciting possibilities for future applications, particularly in targeted alpha-therapy for cancer treatment. From its discovery to its potential in medicine, astatine’s story underscores the importance of continuing to explore the mysteries of the periodic table.
FAQ
- What is the symbol for astatine? (At)
- What is the atomic weight of astatine? (Approximately 210)
- Why is astatine so rare? (Its short half-life makes it decay quickly.)
- How is astatine produced? (By bombarding bismuth with alpha particles.)
- What are the potential medical applications of astatine? (Targeted alpha-therapy for cancer treatment.)
- Why is astatine considered a halogen? (Due to its electron configuration and chemical properties.)
- What is the most stable isotope of astatine? (Astatine-210)
Need more help? Contact us at Phone Number: 0989060241, Email: [email protected] Or visit us at: Tở 2, ấp 5, An Khương, Hớn Quản, Bình Phước, Việt Nam. We have a 24/7 customer support team.